Even though the principal target of freeze-drying is humidity removal, guaranteeing the moment amounts left guiding—residual humidity—are inside acceptable limitations is very important. Why? This seemingly insignificant humidity can have a substantial effect on product security and longevity.
Multi-element mixtures which never crystallize and don't have a eutectic place. They turn into a ‘glass.’
A brand new study located a connection in between processed red meat and dementia possibility. The saturated Extra fat and preservatives in processed meats may possibly contribute to this…
Outside of the biological strengths lyophilization may also aid sustainability. In the present very collaborative scientific Group there is a escalating need to have to transport biological samples. Liquid nitrogen or dry ice is not really required with the storage of freeze-dried samples, which noticeably cuts down shipping and storage expenses (Lewis et al.
The opportunity to renovate drug products into a dry powder without compromising their structural integrity is especially important for preserving The soundness and efficacy of biologic products, like vaccines, antibodies, together with other protein-based mostly therapies.
Although you'll find a myriad of tools and tactics to conduct, the underneath is surely an Over-all manual on the lyophilization process, and a number of the ways required for fulfillment.
, are highlighted together with tips to get more info mitigate them. Last but not least, present tactic for scale-up are shared in conjunction with next-generation PAT applications and methods which could impart meaningful benefit above traditional techniques for cycle enhancement and scale-up and/or enhance The existing approaches.
Exact temperature Management throughout the lyophilization cycle is vital. Both equally freezing and drying temperatures needs to be diligently monitored and managed to forestall products collapse, degradation, or development of analogous products.
As comprehensive higher than, lyophilization needs a complicated freeze-drying process that converts the original pharmaceutical Resolution to the final powdered “cake” that is steady and in a position to be reconstituted later on.
Do you may have questions about the installation of a freeze dryer? Or would you prefer to understand more details on Demaco’s products and companies? Feel free to Speak to us or take a look at our products and assignments.
As soon as the controlled freezing phase sets the phase, we transition to the drying phases, which are split into two major phases, primary and secondary drying:
In the secondary or last drying stage, the residual humidity material is lessened just as much as you possibly can to make check here certain that the product or service is inside a permanently storable point out. The water bound by adsorption at The interior surface of your solution has to be taken off. To realize this, it is often required to prevail over h2o’s capillary forces.
have one or more cryogenic tanks, generally known as dewars, located inside of or outside the house their building. These dewars include the essential liquid nitrogen.
Sure h2o stays within the vial as it truly is fixed to The interior composition by Unique bonds. Interstitial fluids now lie in between the ice crystals and variety a constant net that contains each of the compounds inside of the initial technique.
 Tia Carrere Then & Now!
Tia Carrere Then & Now! Michael Jordan Then & Now!
Michael Jordan Then & Now! Mike Vitar Then & Now!
Mike Vitar Then & Now! Lynda Carter Then & Now!
Lynda Carter Then & Now!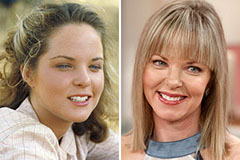 Melissa Sue Anderson Then & Now!
Melissa Sue Anderson Then & Now!